Lung cancer remains one of the most prevalent and deadly cancers worldwide, with millions of deaths each year. Among its various subtypes, lung adenocarcinoma (LUAD) stands out due to its aggressive nature and poor treatment outcomes. Despite significant advancements in early detection and therapeutic strategies, the prognosis for LUAD patients often remains unfavorable. This grim outlook is largely attributed to the intricate molecular mechanisms that drive the progression and resistance of the disease.

A key molecular mechanism that garnered significant attention is N6-methyladenosine (m6A) RNA methylation. m6A is a common and dynamic RNA modification that plays a critical role in several aspects of RNA metabolism, including its stability, splicing, and translation. In the context of cancer, including LUAD, m6A methylation has been implicated in crucial processes such as tumorigenesis, metastasis, and resistance to chemotherapy. This makes m6A a compelling target for novel therapeutic interventions.
A recent study published in Cell Death Discovery explores the role of m6A in LUAD, with a particular focus on the m6A methyltransferase complex, including METTL14. The researchers aimed to elucidate how alterations in m6A methylation contribute to LUAD's aggressive nature and poor patient outcomes. They began by analyzing m6A methylation patterns in LUAD tissue samples compared to normal lung tissues. This analysis was critical in identifying differences in m6A modification levels between cancerous and non-cancerous tissues.
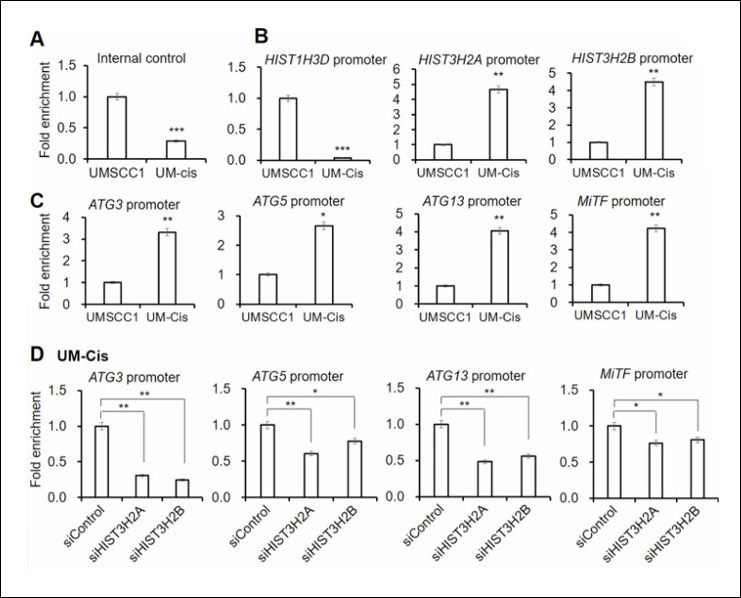
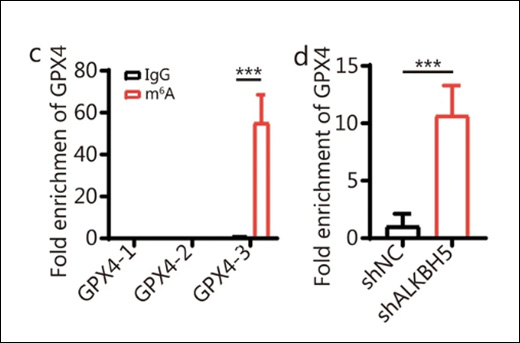
The study also explored the role of the m6A methyltransferase complex in LUAD by assessing the expression levels of key components such as METTL3, METTL14, and WTAP. A significant finding was that m6A methylation regulates glucose-6-phosphate dehydrogenase (G6PD), an enzyme that helps convert glucose into energy and protects cells from damage. The researchers found that METTL14-mediated m6A modification increased the stability and translation of G6PD mRNA, leading to elevated G6PD expression in LUAD tissues. Higher G6PD levels were associated with more aggressive forms of LUAD, underscoring the crucial role of m6A in regulating this enzyme.
To accurately quantify m6A levels in the RNA extracted from these tissue samples, the researchers utilized EpigenTek's EpiQuik™ m6A Methylation Quantification Kit. This advanced tool allowed the researchers to measure m6A levels with high precision and sensitivity. The data obtained demonstrated a significant correlation between elevated m6A levels and more aggressive forms of LUAD, validating the role of m6A in cancer progression.
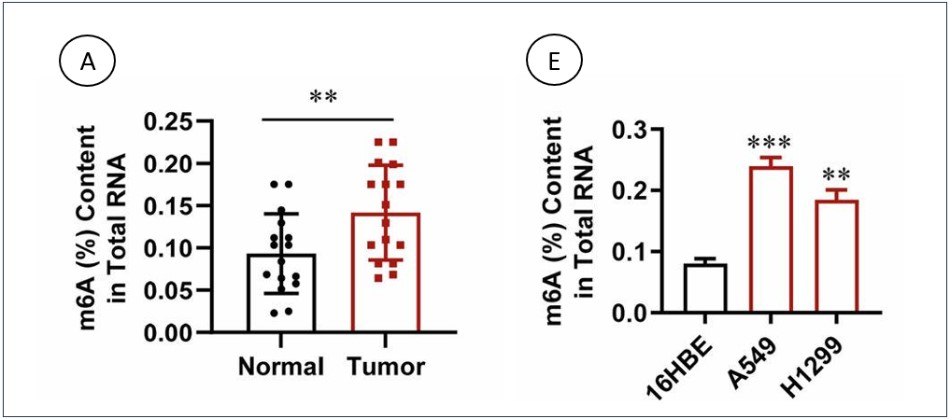
The findings from this study have substantial implications for the future of LUAD treatment. By clarifying the role of m6A and its regulatory proteins in LUAD, the research opens up new avenues for targeted therapies. These potential treatments could involve modulating m6A methylation to inhibit LUAD growth and metastasis, offering new hope for patients. The ability to accurately measure m6A levels using tools like the EpiQuik™ m6A Methylation Quantification Kit is essential for advancing research and developing effective therapeutic strategies.
In summary, m6A methylation is crucial for understanding LUAD and other cancers. The insights from this research, along with advanced tools, pave the way for new strategies to tackle these challenging diseases. As our knowledge of m6A deepens, it is expected to significantly influence the development of future therapies for LUAD and potentially other cancers.
Source: Wu W et al. (August 2024). METTL14-mediated m6A mRNA modification of G6PD promotes lung adenocarcinoma. Cell Death Discov. 10(1):361.




 Cart (0)
Cart (0)