Benign prostatic hyperplasia (BPH) is a common condition in aging men, marked by prostate enlargement and increased fibrosis (collagen buildup). Advanced fibrosis often reduces the effectiveness of treatments, yet the molecular drivers of this process remain unclear.
In a study published in Military Medical Research, researchers uncovered a surprising link between bacterial infection, RNA methylation, and tissue fibrosis, revealing a microbial–epigenetic pathway contributing to disease progression.
What the Researchers Wanted to Find
The team observed that prostate tissues from patients with more fibrosis had higher levels of Salmonella enterica. This raised an important question: could bacterial infection promote fibrosis through an epigenetic pathway?
They focused on ALKBH5, an m6A RNA demethylase. m6A (N6-methyladenosine) is a chemical modification on RNA that affects mRNA stability and translation. If Salmonella induced ALKBH5 activity, it could reduce m6A levels on key transcripts, destabilizing them and triggering oxidative stress and cell injury leading to fibrosis.
How They Conducted the Study
Researchers treated human prostate stromal cells (WPMY-1) with lipopolysaccharide (LPS) from Salmonella enterica (S.e-LPS) and confirmed that this induced fibrosis-like changes: increased collagen deposition, contractility, oxidative stress, and ferroptosis, a type of iron-dependent cell death.
They then examined the role of ALKBH5 using knockdown experiments in vitro and in animal models. Reducing ALKBH5 expression reversed many of the fibrosis-associated changes, suggesting its involvement in the pathway.
Using EpigenTek’s MeRIP Kit to Measure m6A
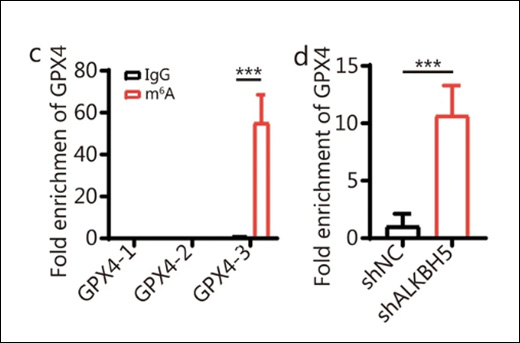
To confirm how Salmonella affected RNA methylation, the researchers used EpigenTek’s EpiQuik™ CUT&RUN m6A RNA Enrichment (MeRIP) Kit to analyze m6A levels on GPX4 mRNA. The assay allowed them to precisely capture and measure methylated RNA, showing that S.e-LPS exposure reduced m6A modification on GPX4, leading to transcript instability and decreased gene expression.
Key Findings
The study found that S.e-LPS exposure increases ALKBH5 expression, which removes m6A methylation from GPX4 mRNA. The loss of this modification destabilizes GPX4 transcripts, reducing GPX4 protein levels and weakening the cell’s antioxidant defenses. As a result, lipid peroxidation and ferroptosis occur, promoting fibrosis in prostate tissue. Importantly, knocking down ALKBH5 restored GPX4 expression, reduced ferroptosis, and reversed fibrosis-related changes in both cell and animal models.
Why It Matters
This study uncovers a new link between bacterial infection and gene regulation in prostate disease. The researchers found that Salmonella can trigger a chain reaction—raising ALKBH5 levels, which removes chemical marks (m6A methylation) from the GPX4 gene. This change weakens the cell’s natural defenses, leading to cell damage and tissue scarring (fibrosis) seen in prostate enlargement. The findings show how infections can alter gene activity through epigenetic mechanisms and suggest new ways to prevent or treat advanced BPH and other fibrosis-related conditions.
Supporting Epitranscriptomic Research with EpigenTek
This work demonstrates the power of m6A profiling in uncovering disease mechanisms. EpigenTek’s MeRIP m6A provided the sensitivity and specificity needed to detect RNA methylation changes on GPX4 mRNA, enabling the discovery of this pathogenic pathway.
EpigenTek offers a full suite of RNA epigenetics solutions, including global RNA methylation kits, transcriptome-specific RNA methylation kits, and RNA methylation antibodies—helping researchers explore how RNA modifications drive health and disease.




 Cart (0)
Cart (0)