Plants are constantly challenged by heat, drought, and other stresses—but they can’t move to escape. Instead, they rely on precise internal communication to adjust and recover. One critical part of this system is the endoplasmic reticulum (ER), which folds and assembles new proteins. When the ER becomes overloaded, misfolded proteins accumulate and trigger endoplasmic reticulum stress (ER stress).
To survive, plants activate the unfolded protein response (UPR), which increases protective chaperones to restore protein-folding balance. Interestingly, many UPR-related genes are active at specific times of day, suggesting they are controlled by the circadian clock. Yet, the exact link between the clock and stress recovery has remained unclear.
What the Researchers Wanted to Know
Scientists suspected that the clock gene CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) might be a master regulator that times the plant’s response to ER stress. If true, CCA1 should directly bind stress-response genes and activate them.
The team set out to determine if CCA1 physically binds the DNA of UPR genes to switch them on during ER stress. To answer this, they needed a method sensitive enough to detect protein–DNA interactions from small amounts of plant tissue—and precise enough to reveal exactly where on the genome CCA1 binds.
Using CUT&RUN-Fast
Traditional chromatin immunoprecipitation (ChIP) methods are often challenging in plants, requiring large sample amounts and producing high background noise. To overcome these hurdles, the researchers turned to CUT&RUN (Cleavage Under Targets and Release Using Nuclease)—a newer technique that maps protein–DNA interactions with much higher sensitivity and resolution.
Using the EpiNext™ CUT&RUN-Fast Kit from EpigenTek, they were able to profile CCA1’s chromatin binding directly from nuclei with low input and minimal background noise, making it ideal for their Arabidopsis thaliana samples.
First, whole seedlings were grown and exposed to ER stress. The team isolated nuclei from ground seedling tissue and used the kit with an anti-CCA1 antibody to perform CUT&RUN, then analyzed the recovered DNA fragments by qPCR.
As complementary validation, they also performed ChIP–qPCR using a purified anti-CCA1 antibody to confirm CCA1 binding to the BiP3 promoter at two sites (R2 and R6).
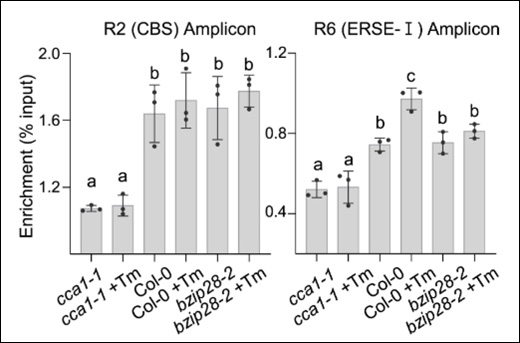
The overall results of the study showed that CCA1 doesn’t just follow the circadian clock—it helps set the timing of stress recovery itself. Plants lacking CCA1 were far more sensitive to stress and recovered poorly, confirming its crucial role in coordinating UPR gene activation during ER stress.
How CUT&RUN-Fast Made It Possible
Precisely mapping CCA1’s binding sites was essential to uncovering its role in stress recovery, and this was achieved using the EpiNext™ CUT&RUN-Fast Kit. The kit provided a fast, low-input, and low-background method that allowed the researchers to clearly detect CCA1’s chromatin binding activity, even from challenging plant tissue.
Their findings demonstrate how Cleavage Under Targets and Release Using Nuclease (CUT&RUN) can drive breakthrough discoveries in plant gene regulation, helping reveal the chromatin-level mechanisms that support stress resilience.
EpigenTek offers a complete suite of CUT&RUN-based solutions—including the EpiNext™ CUT&LUNCH Assay Kit for efficient chromatin profiling and the EpiNext™ CUT&LUNCH Library Preparation Kit for streamlined downstream library construction—empowering researchers to explore chromatin-level gene regulation and uncover new insights into stress resilience and beyond.




 Cart (0)
Cart (0)