Cisplatin-based chemotherapy remains a cornerstone treatment for oral squamous cell carcinoma (OSCC), the most common form of head and neck cancer. Yet, many tumors eventually develop resistance, making therapy less effective. Increasing evidence suggests that chromatin accessibility—how tightly or loosely DNA is packaged within the nucleus—plays a key role in determining how cancer cells respond to stress and treatment. By altering chromatin structure, cells can activate survival pathways such as autophagy, enabling them to withstand chemotherapy.
Study Goals
In a study published in Cell Death & Disease, researchers investigated how chromatin remodeling contributes to cisplatin resistance in OSCC. By comparing cisplatin-sensitive UMSCC1 cells with cisplatin-resistant UM-Cis cells, the team explored how histone gene expression influences chromatin accessibility and triggers autophagy activation. Their goal was to uncover an epigenetic mechanism that helps cancer cells escape the effects of chemotherapy.
Approach and Use of EpigenTek Kit
To evaluate chromatin structure, the researchers used the EpiQuik™ Chromatin Accessibility Assay Kit, which measures DNA openness through nuclease digestion followed by qPCR. This assay provided a precise, quantitative method to assess chromatin compaction at promoter regions of autophagy-related genes, revealing how structural differences in DNA packaging contribute to chemoresistance.
Key Results
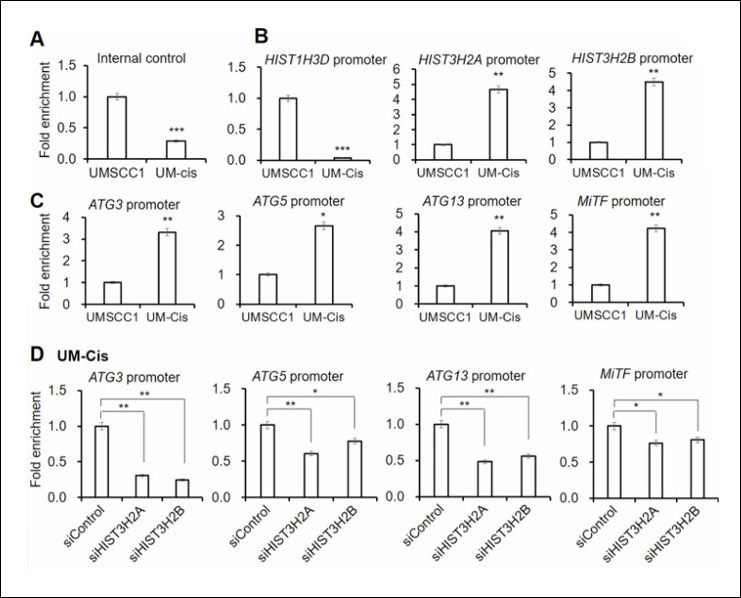
Using EpigenTek’s kit, the researchers found that UM-Cis cells exhibited greater chromatin accessibility at autophagy gene promoters than UMSCC1 cells, shown by larger Ct shifts between digested and undigested DNA. This increased accessibility correlated with higher expression of ATG family genes and elevated autophagy activity—directly linking open chromatin states to drug resistance.
Main Findings
Two histone genes, HIST3H2A and HIST3H2B, were identified as key drivers of this process. Their overexpression promoted chromatin relaxation and autophagy activation in resistant cells. Silencing these genes led to chromatin compaction, reduced autophagy, and restored cisplatin sensitivity. Tumor samples from OSCC patients showed similar patterns, reinforcing the clinical relevance of these findings.
Why It Matters
This research provides compelling evidence that histone-mediated chromatin remodeling enables cancer cells to survive chemotherapy by sustaining autophagy. Targeting the chromatin–autophagy axis could open new therapeutic avenues to re-sensitize resistant tumors and improve outcomes in patients with OSCC and other cancers treated with cisplatin. Using EpigenTek’s EpiQuik™ Chromatin Accessibility Assay Kit, the study demonstrated that histone-driven chromatin decompaction enhances autophagy and promotes cisplatin resistance in oral cancer. These results highlight how epigenetic regulation of chromatin accessibility shapes gene activity and influences cancer treatment response.
How EpigenTek Supports This Research
EpigenTek provides complete solutions for studying chromatin and histone regulation. In addition to chromatin accessibility tools, our chromatin extraction kits, in vivo protein-DNA interaction kits, and histone modification tools enable comprehensive analysis of chromatin structure and histone mark dynamics to better understand gene regulation and epigenetic alterations in cancer.



 Cart (0)
Cart (0)