Research led by the U.S. Food and Drug Administration (FDA), the University of Oxford, and the University of Dundee has revealed that delivering CRISPR-Cas9 tools into the body can trigger immune reactions capable of undermining gene therapy outcomes. Published in Molecular Therapy: Methods & Clinical Development, the work provides direct evidence that AAV-mediated Cas9 delivery can activate cytotoxic T cells that attack edited cells.
Why Cas9 Delivery Matters
CRISPR-Cas9 is widely regarded as a transformative tool for correcting genetic diseases. For systemic disorders that affect multiple organs, researchers often rely on adeno-associated virus (AAV) vectors to carry Cas9 into the body. The Staphylococcus aureus Cas9 (saCas9) variant is particularly attractive for this purpose because of its compact size, which allows it to fit inside AAV’s limited packaging capacity. However, Cas9’s bacterial origin raises concerns about how the human immune system may respond once the enzyme is expressed in cells.
How the Study Was Performed
To explore whether CRISPR delivery could trigger an immune response, the researchers first created an AAV2 viral vector carrying the saCas9 gene and introduced it into human A375 cells. This allowed the cells to begin producing the Cas9 protein, mimicking how gene therapy would work inside the body.
To verify Cas9 expression, the team used EpigenTek’s EpiQuik™ CRISPR/SaCas9 (S. aureus) Assay ELISA Kit, a quantitative assay designed to precisely measure the amount of Cas9 protein inside cells. Once protein production was confirmed, the scientists applied immunopeptidomics, an advanced mass spectrometry-based technique that identifies protein fragments displayed on the surface of cells by human leukocyte antigen (HLA) molecules. These fragments, or “epitopes,” are what the immune system scans to distinguish self from foreign — making them key to understanding whether Cas9 might be targeted as a threat.
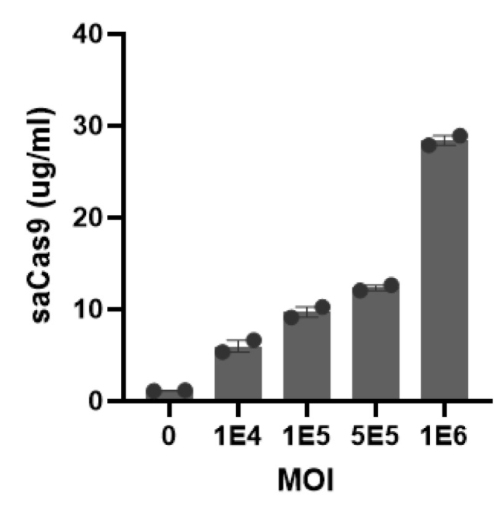
Key Findings
The experiments showed that a small fragment of the Cas9 protein — the peptide GLDIGITSV — was consistently presented on the surface of cells by the HLA-A*02:01 molecule. This is highly relevant because HLA-A*02:01 is one of the most common human leukocyte antigen (HLA) gene variants, found in nearly half of people in the U.S. and U.K. HLAs are proteins found on the surface of most cells in your body. They act like “ID cards,” showing small protein fragments (called peptides) to your immune system.
When blood samples from HLA-A*02:01-positive donors were tested, CD8⁺ T cells (the immune system’s “killer” cells) recognized this peptide as foreign. They responded by releasing inflammatory signals, such as interferon gamma (IFN-γ), and directly destroyed the Cas9-expressing cells. Importantly, this reaction was not isolated to one or two individuals — it was observed across multiple donors, demonstrating that this immune response is both consistent and broadly relevant.
Implications for CRISPR Therapies
These findings underscore a critical challenge for CRISPR therapeutics. While AAV-delivered Cas9 allows efficient gene editing, it may also provoke immune responses that eliminate the very cells intended for correction. Future strategies will need to focus on engineering Cas9 variants that are less likely to cause an immune reaction, using short-term immune suppression during treatment, or exploring non-viral delivery systems. Such approaches will be vital for ensuring both safety and long-term effectiveness of in vivo CRISPR therapies.
Summary and Implications
This study shows that AAV-delivered Cas9 is not invisible to the immune system. By combining immunopeptidomics with tools like the EpigenTek CRISPR-SaCas9 ELISA Kit, the researchers demonstrated that Cas9 can generate a potent cytotoxic T cell response, leading to destruction of gene-edited cells. The central lesson: immune recognition is not a side concern but a core hurdle for CRISPR therapies. Overcoming this barrier will be essential for realizing the full therapeutic promise of genome editing.
If you’re interested in studying CRISPR or investigating its immune interactions, EpigenTek’s EpiQuik™ CRISPR/SaCas9 (S. aureus) Assay ELISA Kit offers precise measurement of Cas9 expression in cells and tissues, while the CRISPR/Cas9 Monoclonal Antibody [7A9] provides a dependable tool for detecting Cas9 in genomic editing research. Together, these resources can help support deeper investigations into CRISPR function and immune response.
If you’re interested in studying CRISPR or its immune interactions, we offer a range of CRISPR/Cas9 products, including ELISA kits and antibodies to support your research. Contact us with any questions or pricing details.




 Cart (0)
Cart (0)