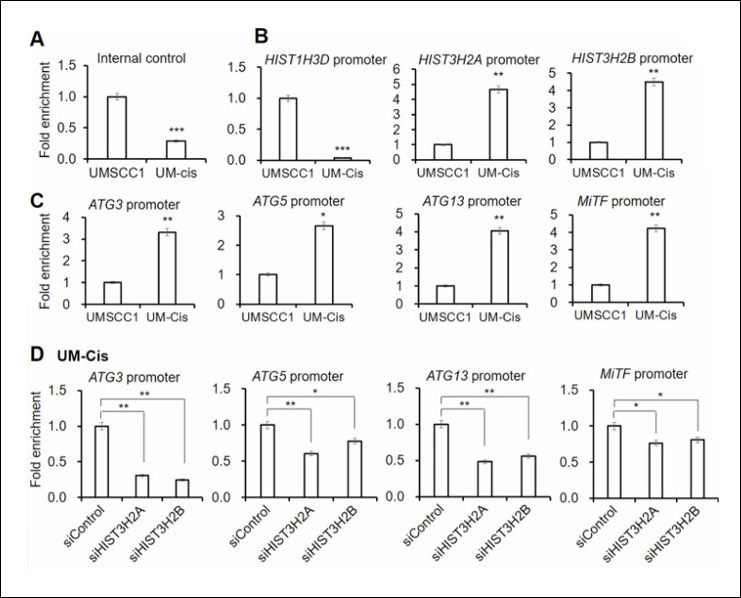
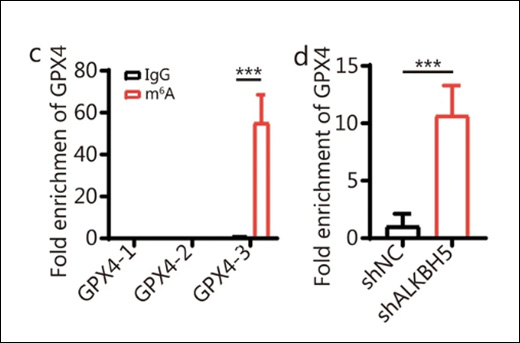
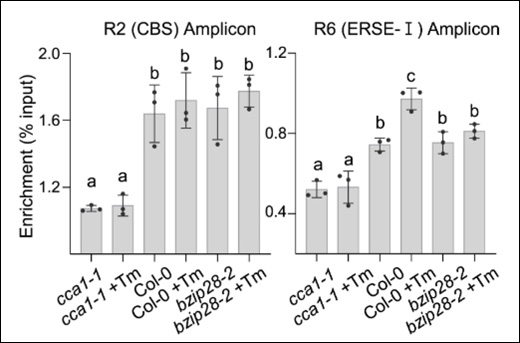
 Epigenetics is a field of biology that explores heritable changes in gene function that do not involve alterations to the DNA sequence itself. These changes steer gene expression and can influence various biological processes, from development to disease susceptibility.
One of the most widely studied epigenetic mechanisms is DNA methylation—the addition of a methyl group to the 5th carbon of cytosine bases within the DNA molecule, forming 5-methylcytosine (5-mC).
Epigenetics is a field of biology that explores heritable changes in gene function that do not involve alterations to the DNA sequence itself. These changes steer gene expression and can influence various biological processes, from development to disease susceptibility.
One of the most widely studied epigenetic mechanisms is DNA methylation—the addition of a methyl group to the 5th carbon of cytosine bases within the DNA molecule, forming 5-methylcytosine (5-mC).
5-mC is a key modification that regulates gene expression without changing the underlying genetic code. The levels and configurations of 5-mC are tightly controlled, and disruptions to normal methylation patterns are associated with various health issues, including metabolic disorders, neurodegenerative diseases, and cancer [1 ,2].
DNA Methylation and Disease
Aberrant DNA methylation patterns—either hypermethylation or hypomethylation—can lead to dysregulated gene expression. Hypermethylation of promoter regions often silences tumor suppressor genes, contributing to cancer progression, while hypomethylation may activate oncogenes or other normally silenced genomic regions, promoting unchecked cell growth. DNA methylation is also implicated in neurodegenerative diseases like Alzheimer’s, cardiovascular diseases, and autoimmune conditions, where gene-environment interactions play a critical role.
Effects of Environment and Lifestyle
Environmental and lifestyle factors have a significant impact on DNA methylation, making it a dynamic interface between our genes and the environment. Environmental exposures and lifestyle choices can alter DNA methylation patterns, leading to changes in gene expression and, ultimately, health outcomes.
Chemical Exposure
Exposure to environmental toxins, such as air pollutants, industrial chemicals, and pesticides, can induce changes in DNA methylation. For example, exposure to heavy metals like cadmium, arsenic, and lead has been tied to abnormal DNA methylation patterns, potentially leading to increased cancer risk [3]. Chemical pollutants, such as bisphenol A—a common plasticizer—have been shown to disrupt DNA methylation and shape developmental processes in fetuses [4].
Chronic exposure to particulate matter in air pollution is linked to changes in the methylation landscape in genes involved in inflammation and immune response [5]. These changes may contribute to cardiovascular and respiratory diseases, underscoring how environmental factors can directly modulate our epigenome and, in turn, our health.
Diet
Dietary factors have profound effects on DNA methylation. Nutrients like folate, vitamin B12, and methionine serve as essential components in the one-carbon metabolism pathway, which supplies the methyl groups necessary for methylation reactions [6].
A diet deficient in folate can lead to reduced DNA methylation, particularly during early development, which is correlated with neural tube defects in embryos. Conversely, diets rich in methyl donors (like green leafy vegetables) can support healthy DNA methylation patterns. The maternal diet during pregnancy, in particular, is critical in shaping the developing fetus’s epigenome and can affect long-term health outcomes.
A high-fat, Western-style diet has been linked to atypical DNA methylation in genes associated with metabolic disorders like obesity and type 2 diabetes. Animal studies show that high-fat diets induce global DNA dysmethylation, leading to altered expression of genes related to insulin resistance and inflammation [7].
Smoking
Cigarette smoking is one of the most well-studied lifestyle factors affecting DNA methylation. Smoking induces widespread DNA methylation changes, particularly in genes affiliated with cancer and lung disease [8].
In smokers, methylation of key tumor suppressor genes (e.g., p16 and APC) is often increased, leading to gene silencing [9]. This repression can prevent the body from effectively regulating cell division, promoting tumor formation. Even after smoking cessation, some DNA methylation changes persist for years, contributing to long-term cancer risk.
Alcohol Consumption
Chronic alcohol use has been associated with DNA methylation anomalies, especially in liver cells, contributing to the development of liver diseases such as cirrhosis and hepatocellular carcinoma [10].
Excessive alcohol consumption depletes key methyl donors (like S-adenosyl methionine), leading to global DNA hypomethylation [11]. This alteration can disrupt the natural expression of genes involved in cellular homeostasis and promote inflammation, fibrosis, and carcinogenesis in the liver.
Stress and Physical Activity
Psychological stress has been linked to DNA methylation changes, particularly in genes connected with stress response, inflammation, and immunity. Chronic stress may induce methylation of the glucocorticoid receptor gene, leading to altered stress hormone signaling and potentially contributing to mood disorders [12].
Conversely, regular physical activity has been shown to promote healthy DNA methylation patterns, particularly in genes related to inflammation and metabolic regulation, offering a protective benefit against chronic diseases.
Detecting and Quantifying DNA Methylation: Tools for Researchers
As DNA methylation plays such a pivotal role in health and disease, researchers need reliable tools to study these epigenetic changes. The MethylFlash™ Global DNA Methylation (5-mC) ELISA Easy Kit, developed by EpigenTek, is a popular choice for scientists. This kit offers a simple and rapid way to quantify the 5-mC modification in DNA samples using a convenient ELISA-based format. With minimal hands-on time and a user-friendly protocol, the MethylFlash™ kit allows for accurate analysis of global DNA methylation levels across a range of sample types.




 Cart (0)
Cart (0)