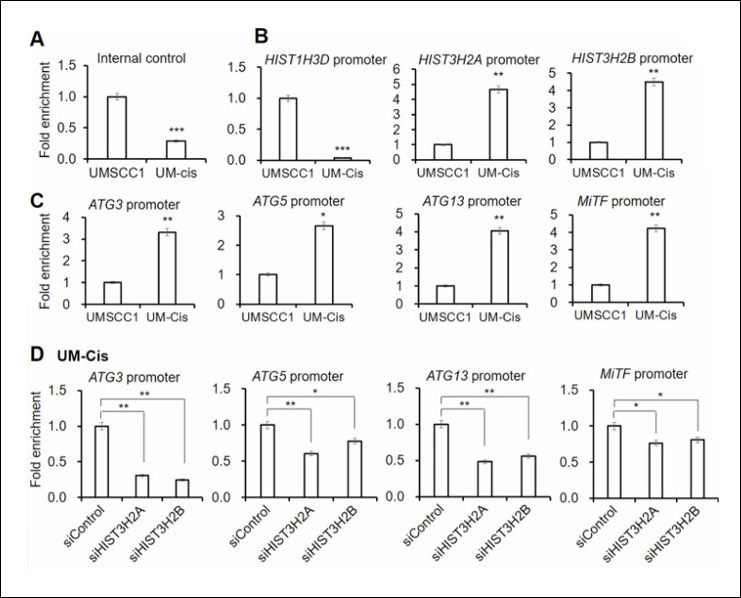
Oh SY et. al. (August 2024). Chromatin remodeling-driven autophagy activation induces cisplatin resistance in oral squamous cell carcinoma Cell Death Dis. 15(8):589.
This study explores how chromatin remodeling drives autophagy activation, leading to cisplatin resistance in oral squamous cell carcinoma (OSCC). By comparing gene expression in cisplatin-sensitive and resistant OSCC cells, researchers found that specific histone genes, like HIST3H2A and HIST3H2B, are linked to increased autophagy and drug resistance. Knockdown of these genes reduced autophagy and restored cisplatin sensitivity. The study also identified potential biomarkers for predicting chemoresistance in OSCC patients, highlighting the interplay between chromatin remodeling and autophagy in drug resistance.
Products Used: EpiQuik Chromatin Accessibility Assay Kit
Ma J et. al. (August 2024). SlWRKY55 coordinately acts with SlVQ11 to enhance tomato thermotolerance by activating SlHsfA2 Plant J.
This study investigates how the transcription factor SlWRKY55 and its partner SlVQ11 enhance tomato thermotolerance by activating the heat stress response gene SlHsfA2. SlWRKY55 directly binds to the promoter of SlHsfA2, boosting its expression, and this effect is amplified by SlVQ11. Both SlWRKY55 and SlVQ11 also interact with SlHsfA2, further increasing its transcriptional activity. This SlWRKY55/SlVQ11-SlHsfA2 pathway reveals a key mechanism in tomato's defense against high temperatures, offering potential targets for improving crop resilience to heat stress.
Products Used: EpiQuik Plant ChIP Kit
Qiu H et. al. (August 2024). Lamin A/C deficiency-mediated ROS elevation contributes to pathogenic phenotypes of dilated cardiomyopathy in iPSC model Nat Commun. 15(1):7000.
This study examines how a frameshift mutation in the LMNA gene, encoding the nuclear envelope protein lamin A/C, contributes to dilated cardiomyopathy (DCM) through elevated reactive oxygen species (ROS). Using patient-specific iPSC-derived cardiomyocytes, the researchers found that lamin A/C deficiency leads to mitochondrial dysfunction, increased ROS levels, and abnormal Ca2+ handling. This triggers the CaMKII-RYR2 pathway and causes nuclear envelope deformation, both of which contribute to arrhythmias in LMNA-related DCM. The findings suggest that targeting SIRT1 degradation and oxidative stress could be promising therapeutic strategies for this condition.
Products Used: Epigenase Universal SIRT Activity/Inhibition Assay Kit (Fluorometric)
Zhu Y et. al. (August 2024). M6A RNA methylation-mediated TUG1 stability maintains mitochondrial homeostasis during kidney aging by epigenetically regulating PGC1-α expression Zhu Y et. al. (August 2024).
This study explores how m6A RNA methylation influences kidney aging by stabilizing the long non-coding RNA TUG1, which is crucial for maintaining mitochondrial homeostasis. The researchers found that in aged kidneys, TUG1 exhibits reduced m6A modification, leading to instability and impaired mitochondrial function. TUG1 directly interacts with and promotes the expression of PGC1-α, a key regulator of mitochondrial quality control. Silencing METTL14, an m6A methylase, or IGF2BP2, a reader protein, disrupts TUG1 stability, contributing to renal aging and fibrosis. This work highlights TUG1 as a potential therapeutic target for mitigating kidney aging.
Products Used: EpiQuik m6A RNA Methylation Quantification Kit (Colorimetric)
Hou X et. al. (August 2024). H3K36 methyltransferase SMYD2 affects cell proliferation and migration in Hirschsprung's disease by regulating METTL3 J Cell Physiol. :e31402.
This study investigates the role of the H3K36 methyltransferase SMYD2 in Hirschsprung's disease (HSCR), a condition affecting the colon's neural crest cells. The researchers found that SMYD2 is the key factor behind differential H3K36 methylation in HSCR, impacting cell proliferation and migration. SMYD2 was also shown to regulate METTL3 expression, thereby influencing m6A RNA methylation levels. This regulation affects the development of the intestinal nervous system in HSCR. The findings highlight the significance of H3K36 methylation and SMYD2 in the disease's pathogenesis, offering new insights into potential therapeutic targets.
Products Used: EpiQuik m6A RNA Methylation Quantification Kit (Fluorometric)
Ying Y et. al. (August 2024). ERP29 regulates the proliferation of endometrial carcinoma via M6A modification Life Sci. :122976.
This study investigates the role of endoplasmic reticulum protein 29 (ERP29) and m6A RNA modification in endometrial cancer (EC). The researchers identified ERP29 as a key endoplasmic reticulum stress (ERS)-related gene highly expressed in EC. They found that m6A modifications, regulated by METTL3, control ERP29 expression and, consequently, EC cell proliferation. Knockdown of ERP29 inhibited cell proliferation, while manipulating m6A levels altered ERP29 expression and affected cancer growth. These findings suggest that ERP29 and m6A modifications could serve as potential diagnostic and therapeutic targets in endometrial cancer.
Products Used: EpiQuik CUT&RUN m6A RNA Enrichment (MeRIP) Kit




 Cart (0)
Cart (0)