Effective regulation of gene expression lies at the core of cellular function and overall organismal health. While transcriptional control mechanisms have long been recognized, attention has increasingly focused on the significance of post-transcriptional modifications, particularly N6-methyladenosine (m6A) methylation of messenger RNA (mRNA). This modification exerts dynamic control over mRNA fate, influencing processes like translation efficiency, mRNA stability, and subcellular localization.
This understanding of mRNA regulation has recently been enriched by a fascinating new layer: the impact of nitric oxide (NO) on m6A dynamics. Inspired by the known interaction between oxygen (O2) and the iron site of fat mass and obesity-associated protein (FTO), researchers from the University of Illinois Chicago, College of Pharmacy, hypothesized that NO, a versatile signaling molecule, might interact similarly with FTO, potentially modulating its demethylase activity. This hypothesis led to meticulous biochemical assays and molecular analyses aimed at deciphering the direct inhibitory effect of NO on FTO. Their research was published in the journal Redox Biology.
To investigate this hypothesis, the researchers employed EpigenTek’s Epigenase™ m6A Demethylase Activity/Inhibition Assay Kit to probe the interaction between NO and FTO. These experiments confirmed NO's role as an endogenous inhibitor of FTO demethylase activity, disrupting its function in removing m6A marks from mRNA transcripts. Additionally, the team utilized EpigenTek’s EpiQuik m6A RNA Methylation Quantification Kit to quantify the changes in m6A levels induced by NO exposure, providing valuable insights into the global hypermethylation of m6A on mRNA transcripts upon NO treatment.
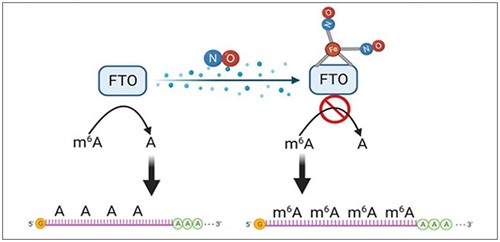
Transcriptomic profiling of NO-treated cells unveiled a subset of mRNA transcripts exhibiting pronounced changes in expression levels correlated with enhanced m6A methylation. These findings shed light on the intricate interplay between NO signaling and mRNA epitranscriptomic regulation. Furthermore, experiments conducted in both in vitro cell culture models and in vivo tumor xenografts confirmed the physiological relevance of NO-mediated FTO inhibition, highlighting the translational potential of targeting the NO-FTO axis in diseases characterized by dysregulated mRNA modification, particularly cancer.
The discovery of NO-mediated epitranscriptomic regulation opens up promising avenues for therapeutic intervention in diseases linked to dysregulated mRNA modification. By focusing on the NO-FTO axis, scientists could devise innovative strategies to rectify abnormal gene expression patterns associated with various pathological conditions, offering a beacon of hope for disease management and treatment.
Overall, the exploration of NO's role in modulating the mRNA epitranscriptome marks a significant stride in our comprehension of cellular regulation. This study not only broadens our understanding of epitranscriptomic regulation but also underscores the potential of targeting the NO-FTO axis for therapeutic intervention in diseases characterized by dysregulated mRNA modification. Further exploration of this newly unearthed mechanism could unlock fresh insights into disease pathogenesis and pave the way for the development of innovative treatment strategies.
Source: Kuschman HP et. al. (October 2023). Nitric oxide inhibits FTO demethylase activity to regulate N(6)-methyladenosine mRNA methylation. Redox Biol. 67:102928.
Kits used:
- Epigenase m6A Demethylase Activity/Inhibition Assay Kit (Colorimetric), #P-9013
- EpiQuik m6A RNA Methylation Quantification Kit, P-9013




 Cart (0)
Cart (0)




