DNA Methylation, a critical epigenetic modification, involves the addition of a methyl group to the 5-carbon position of cytosine residues within CpG dinucleotides, forming 5-methylcytosine, or 5-mC. This modification plays a vital role in gene regulation, development, and disease processes. Abnormal DNA methylation patterns have been implicated in various diseases, including cancer, making accurate measurement of methylation states crucial for both basic research and clinical applications.

Methylated DNA Immunoprecipitation (MeDIP) is a widely used technique to enrich and analyze methylated DNA regions from a genomic sample. The procedure involves the use of antibodies that specifically bind to methylated cytosines, allowing researchers to isolate and study these regions in detail. Despite its effectiveness, the success of MeDIP hinges on several technical considerations that can have a major impact on the reliability and consistency of the results. This guide provides benchtop tips for optimizing MeDIP assays to ensure robust and reproducible outcomes.
Starting Material
High-quality, purified DNA is fundamental for successful MeDIP assays. Contaminants such as RNA and proteins can interfere with the binding of antibodies and compromise the immunoprecipitation step. To verify the purity and integrity of the DNA, use a reliable DNA extraction method (e.g., phenol-chloroform extraction) that yields high-purity DNA free from contaminants. The quality of DNA can be gauged using methods such as agarose gel electrophoresis or a bioanalyzer to confirm the absence of degradation.
Input Amount
The amount of DNA used in the MeDIP assay can significantly affect the outcome. Too little DNA can lead to poor signal detection, while too much DNA can cause non-specific binding and high background noise. Start with a DNA quantity recommended by the specific MeDIP protocol or manufacturer’s guidelines. Generally, 500 ng to 2 µg of DNA is recommended as a starting amount. Carry out a pilot experiment to determine the optimal input for your specific system and antibody.
DNA Shearing
Genomic DNA should be sheared into smaller fragments before starting MeDIP. Proper DNA fragmentation ensures that methylated regions are accessible for antibody binding and helps to avoid biases in the enrichment process. Oversized DNA fragments may reduce targeted DNA capturing via antibody, while undersized fragments may decrease the efficiency of downstream analyses like PCR. Ideal fragment sizes for MeDIP typically range between 200-500 base pairs. This size range allows efficient antibody binding while maintaining an adequate resolution of methylation patterns.
Different shearing options are available, including water bath sonication, probe-based sonication, and enzyme-based approaches. The size of sheared DNA can be verified before starting the immunoprecipitation step by staining with ethidium bromide or a suitable fluorescent dye for DNA on a 1-2% agarose gel and visualizing it under UV light.
Internal Controls
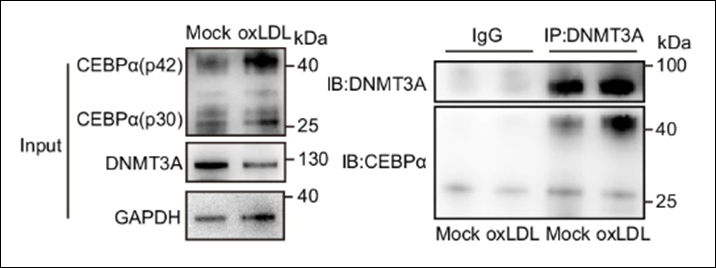
Incorporating internal controls is essential for assessing the specificity and effectiveness of MeDIP assays. Non-immune IgG serves as a negative control to evaluate non-specific binding and the efficacy of wash steps. This control helps to identify background noise and ascertain the purity of the immunoprecipitated DNA. Fully methylated and unmethylated DNA fragments are useful as specificity controls. These controls help to validate the antibody’s ability to distinguish between methylated and unmethylated DNA, ensuring the accuracy of the enrichment.
Antibody Selection
The choice of antibody is another key factor. The antibody should be highly specific to 5-mC and not cross-reactive with other cytosine modifications such as 5-hydroxymethylcytosine (5-hmC) or unmethylated DNA. Choose antibodies validated for MeDIP purposes, preferably with published evidence of their specificity and sensitivity. Validate the antibody with known methylated and unmethylated DNA controls to confirm its specificity.
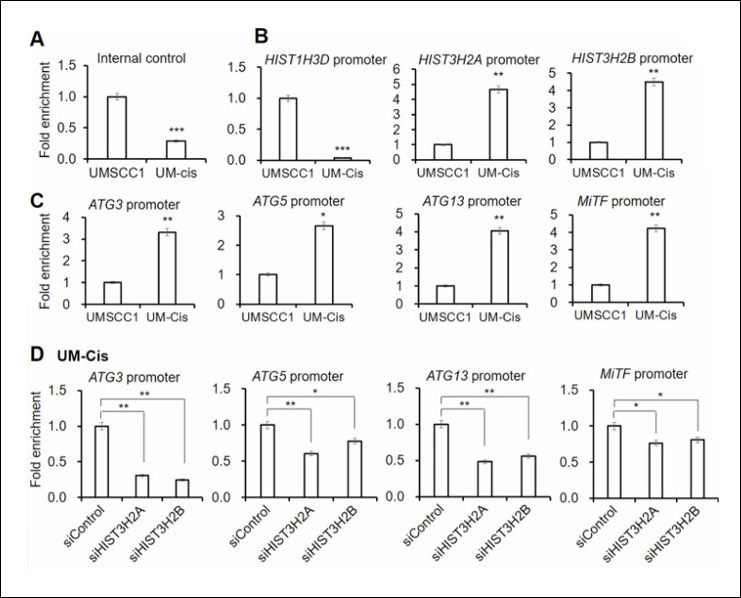
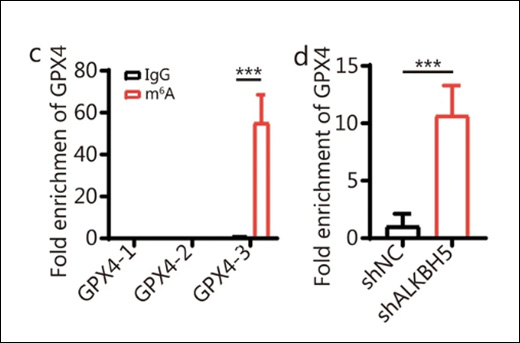
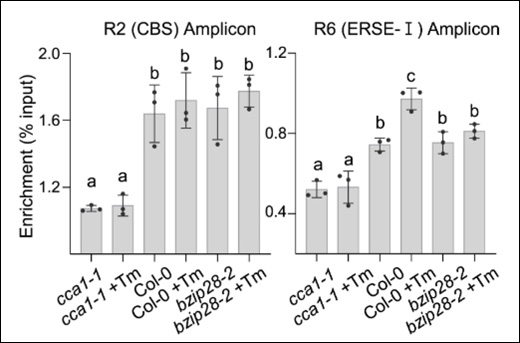
Fold Enrichment
Measuring the fold enrichment of methylated DNA is important for evaluating the performance of the MeDIP assay. Fluorescence-based methods for DNA quantification, such as Qubit, offer higher sensitivity and are less affected by contaminants compared to spectrophotometric methods like NanoDrop. Quantitative PCR can be used to examine the enrichment of specific methylated regions. Fold enrichment can be calculated by simply using a ratio of amplification efficiency of the MeDIP sample over that of non-immune IgG: Fold Enrichment = 2(IgG Ct - Sample Ct)
Quick, Simple, and Reliable MeDIP Assays
As the leading developer and provider of innovative technologies and products for epigenetics-related research, EpigenTek has created a comprehensive suite of MeDIP kits for your research needs. These kits are complete sets of optimized reagents for extremely fast and convenient enrichment of methylated DNA fragments from a variety of sources, like cells, tissues, and isolated genomic DNA. The enriched DNA obtained with these kits is compatible with the most common downstream applications, including qualitative and quantitative PCR, microarray, and sequencing.




 Cart (0)
Cart (0)